Angiotensin 1/2 (1-9)

(Synonyms: 血管紧张素I,H2N-Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-OH ) 目录号 : GP10025
血管收缩药
Cas No.:34273-12-6
Sample solution is provided at 25 µL, 10mM.
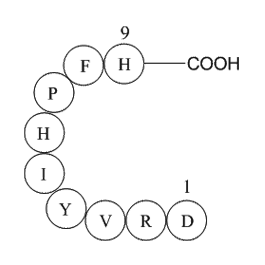
Angiotensin I/II (1-9) is a peptide (ASP-ARG-VAL-TYR-ILE-HIS-PRO-PHE-HIS) containing the amino acids 1-9 that are converted from Angiotensin I/II peptide.
Angiotensin I is formed by the action of renin on angiotensinogen, which has 12 amino acids and is an ǁ-2-globulin produced constitutively and released into the circulation mainly by the liver. Renin cleaves the peptide bond between the leucine (Leu) and valine (Val) residues on angiotensinogen, creating the ten-amino acid peptide angiotensin I. Angiotensin I is converted to angiotensin II (AII) through removal of two C-terminal residues by the enzymeangiotensin-converting enzyme (ACE), primarily through ACE within the lung.
Angiotensin is a peptide hormone that causes vasoconstriction and a subsequent increase in blood pressure. Angiotensin also stimulates the release of aldosterone, which promotes sodium retention in the distal nephron so that drives blood pressure up.
References:
1. Basso N, Terragno NA (December 2001). "History about the discovery of the renin-angiotensin system". Hypertension 38 (6): 1246-9.
2. Richard A. Preston. et. (1998). pAge-Race Subgroup Compared With Renin Profile as Predictors of Blood Pressure Response to Antihypertensive Therapyq. JAMA. 1998;280(13):1168-1172.
3. Williams GH, Dluhy RG (2008). "Chapter 336: Disorders of the Adrenal Cortex". In Loscalzo J, Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL. Harrison's principles of internal medicine. McGraw-Hill Medical.
| Cas No. | 34273-12-6 | SDF | |
| 别名 | 血管紧张素I,H2N-Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-OH | ||
| 化学名 | Angiotensin 1/2 (1-9) | ||
| Canonical SMILES | CCC(C)C(C(=O)NC(CC1=CN=CN1)C(=O)N2CCCC2C(=O)NC(CC3=CC=CC=C3)C(=O)NC(CC4=CN=CN4)C(=O)O)NC(=O)C(CC5=CC=C(C=C5)O)NC(=O)C(C(C)C)NC(=O)C(CCCN=C(N)N)NC(=O)C(CC(=O)O)N | ||
| 分子式 | C56H78N16O13 | 分子量 | 1183.32 |
| 溶解度 | ≥ 118.3mg/mL in DMSO | 储存条件 | Store at -20°C |
| General tips | 请根据产品在不同溶剂中的溶解度选择合适的溶剂配制储备液;一旦配成溶液,请分装保存,避免反复冻融造成的产品失效。 储备液的保存方式和期限:-80°C 储存时,请在 6 个月内使用,-20°C 储存时,请在 1 个月内使用。 为了提高溶解度,请将管子加热至37℃,然后在超声波浴中震荡一段时间。 |
||
| Shipping Condition | 评估样品解决方案:配备蓝冰进行发货。所有其他可用尺寸:配备RT,或根据请求配备蓝冰。 | ||
| 制备储备液 | |||
 |
1 mg | 5 mg | 10 mg |
| 1 mM | 0.8451 mL | 4.2254 mL | 8.4508 mL |
| 5 mM | 0.169 mL | 0.8451 mL | 1.6902 mL |
| 10 mM | 0.0845 mL | 0.4225 mL | 0.8451 mL |
| 第一步:请输入基本实验信息(考虑到实验过程中的损耗,建议多配一只动物的药量) | ||||||||||
| 给药剂量 | mg/kg |  |
动物平均体重 | g |  |
每只动物给药体积 | ul |  |
动物数量 | 只 |
| 第二步:请输入动物体内配方组成(配方适用于不溶于水的药物;不同批次药物配方比例不同,请联系GLPBIO为您提供正确的澄清溶液配方) | ||||||||||
| % DMSO % % Tween 80 % saline | ||||||||||
| 计算重置 | ||||||||||
计算结果:
工作液浓度: mg/ml;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL,
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL saline,混匀澄清。
1. 首先保证母液是澄清的;
2.
一定要按照顺序依次将溶剂加入,进行下一步操作之前必须保证上一步操作得到的是澄清的溶液,可采用涡旋、超声或水浴加热等物理方法助溶。
3. 以上所有助溶剂都可在 GlpBio 网站选购。
Quality Control & SDS
- View current batch:
- Purity: >98.00%
- COA (Certificate Of Analysis)
- SDS (Safety Data Sheet)
- Datasheet





















